This isn’t about beating the odds,
This is about changing them
Our researchers work relentlessly to discover innovative solutions for many of the barriers that our patients face. The knowledge gained through research influences the innovative care we give all our patients at Seattle Children’s.
Award Winning Research at Seattle Children’s
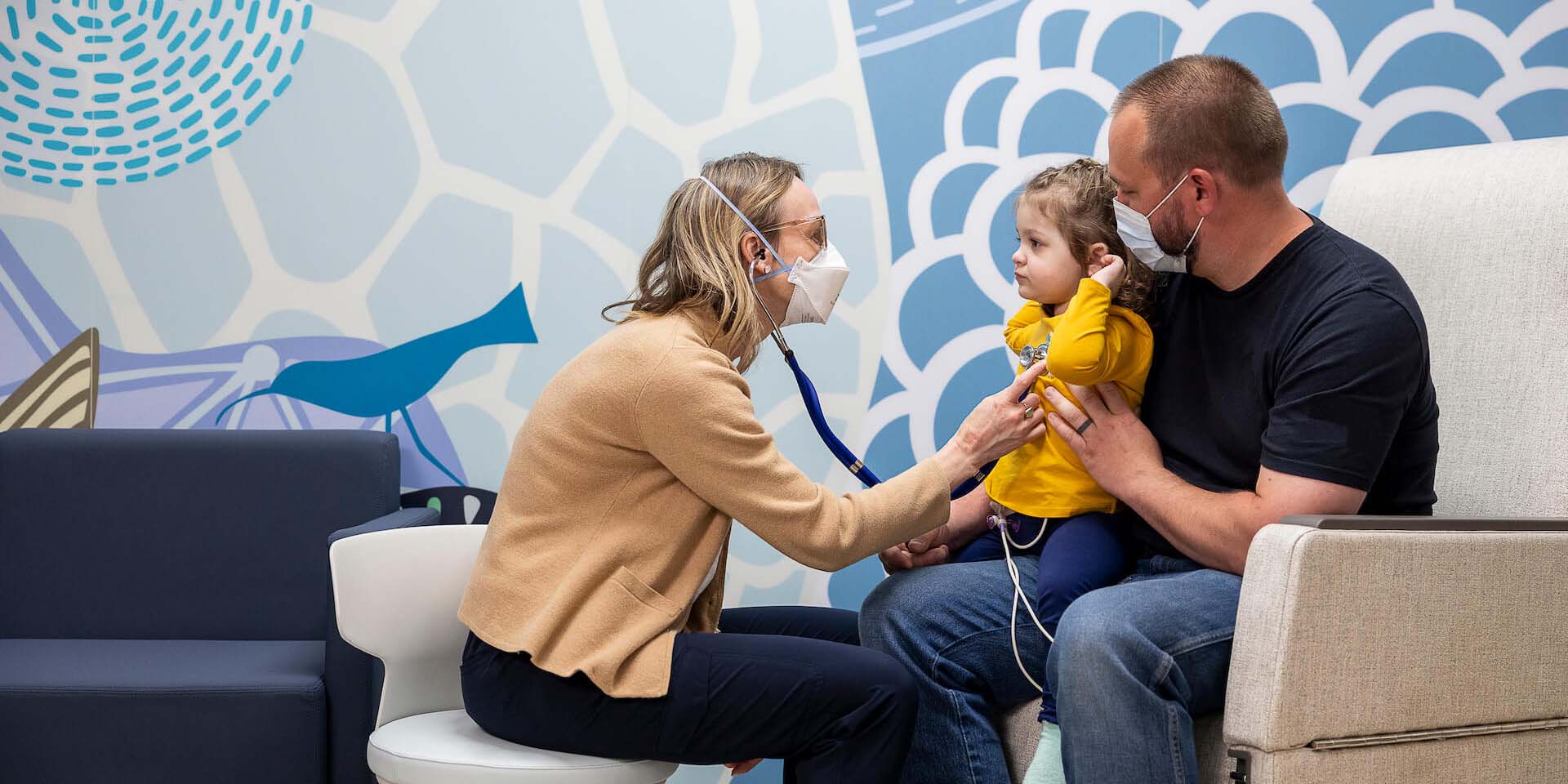
“It was nice to hear right when Rowan was diagnosed that there were clinical trials running and an option for Rowan to be part of one. Just knowing that clinical research was being done and his doctors were involved was encouraging.”
— Erin, mother of Rowan, who participated in a clinical trial for childhood leukemia
Learn More About Dr. Rachel Rau’s Award-Winning Clinical Trial

In 2025, U.S. News & World Report again named Seattle Children’s among the top 10 best children’s hospitals in the U.S. and the best in the Pacific Northwest. For more than 30 years, our teams of dedicated researchers have worked relentlessly to improve treatments for more than 200 diseases and conditions. Together, with your family’s participation, we’re developing breakthrough clinical trials and research studies to discover the most effective, least invasive and best options for the kids who need it most.

Clinical Trials and Research Studies at Seattle Children's
Clinical trials are research studies that test whether a new medicine, device or treatment is safe and effective. Research studies can help find new and better ways to understand, detect, control and treat illnesses and other medical conditions.
Many children, adolescents and young adults with various conditions travel from all over the world to take part in research studies of new investigational options only available through Seattle Children’s Research Institute.
Research Participant Stories
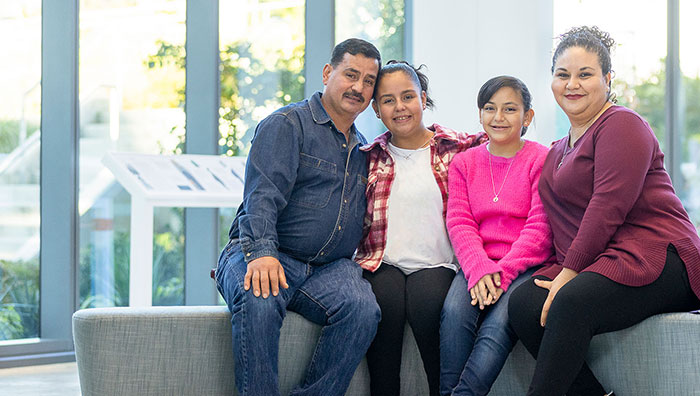
“I was nervous, but then Maria walked into the room, and she started speaking my language ... There was no middle person there, so I felt like I was getting clear information from her, and she was getting clear information from me.”
— Ana, whose two daughters participated in Seattle Children's research studies

“I don't have the words to express what Children’s commitment to research means. Many kids, not only from all over the United States, but from all over the world, don't have this access. Especially for the DIPG community, Seattle Children's is one of the top places to be. We're so lucky it’s so close for us.”
— Ashlee, mom of Gavin who participated in groundbreaking CAR T-cell trial to treat lethal brain tumor
Clinical Trial and Research Study Frequently Asked Questions
Clinical research helps doctors and researchers find new and better ways to understand, detect, control and treat illness and other medical conditions. Clinical research involves carefully planned studies that people volunteer to take part in. They help researchers learn more about health and find new medications and treatments. There are 2 main types of studies:
- Interventional studies (also called clinical trials) test new medicines and treatments in people to see how well they work and to make sure they are safe.
- Observational studies collect information about health and behavior. This is done through surveys, interviews or observing study participants over time.
Before a new medication or treatment can become a standard practice of care it must go through research phases.
Each research phase must:
- Answer a research question
- Meet safety rules before the next phase takes place
- Meet phase goals before the next phase takes place
It is useful to know what phase the study is in to help you understand the goals of the research. A person from the research team will be able to tell you this information.
- Phase 1: Researchers test a new medicine or treatment in a small group of people for the first time to see if it is safe, how it will be given and if there are side effects.
- Phase 2: The medicine or treatment is given to a slightly larger group of people to study if it is effective and further study its safety. Sometimes side effects happen to only a few people. This is why testing on a larger number of people is important.
- Phase 3: The medicine or treatment is given to large groups of people to confirm effectiveness and compare it to other already approved medicines or treatments.
- Phase 4: After the medicine or treatment has been approved for use, often more research is done to look for effectiveness in various groups of people (for example, in patients age 12 and older) and to see if there are side effects associated with long-term use.
Safety is our main priority at Seattle Children’s. All studies follow strict regulations and guidelines:
- We have an ethics board within Seattle Children’s that reviews studies to protect children who take part. This is called an institutional review board (IRB).
- The U.S. Food and Drug Administration (FDA) must approve the design of a study before any new medicine, treatment or device is tested with people.
- Some studies have added layers of protection. For example, the National Cancer Institute (NCI) monitors many of the studies we offer. If participants from another hospital in the study have side effects from the study treatment, NCI reports this information to us.
Before your child joins a study, the research team will explain any risks of participating. The potential risks and potential benefits are listed on the consent and assent documents that you and your child will review with someone from the research team before joining a study.
Taking part in research can look a lot like the regular medical care your child receives at a doctor’s office, clinic or hospital. Sometimes doctors also do research. It is important to talk with your doctor and the research team about the differences between the research and your child’s regular medical care.
In medical care, the doctor or your care team develops a plan of care tailored for your child.
When your child takes part in a study, the research team follows a set plan called a study protocol. The goal of research is to add to medical knowledge that might lead to improved treatments for children in the future. Your child might or might not benefit directly from being in a research study.
Learn more on the How to Participate in a Research Study page.
Every study has a lead researcher called a principal investigator (PI), who is often a medical doctor. They oversee the research study. Some studies also have a research team that might include doctors, nurses, social workers, research coordinators and other healthcare professionals. The PI and research team closely monitor a participant’s health throughout the study.
Being in a study is voluntary. Your child can stop participating in a study at any time without it affecting your regular medical care at Seattle Children’s.
You might be asked to fill out a survey to share why you decided to stop taking part in the study. Filling out the survey is voluntary.
Learn About Clinical Trial Options
We're Here to Help
We understand there’s a lot to consider when searching for the right clinical trial. That’s why we’re here to keep you informed and help however we can.
Interpreter Services Available | Servicios de intérprete disponibles | Có Dịch vụ Thông dịch
844-226-4886
Call 206-884-1156. (English)
